BIOFIRE® FILMARRAY®
The Fastest Way to Better Results
User-friendly Multiplex PCR
The FILMARRAY System has set a new standard in molecular diagnostic platforms featuring:
- Unmatched usability and speed
- Comprehensive panels
- Results in about 60 minutes
- Physicians get answers sooner
- Laboratories maximize productivity and reduce costs
For More
Information
The Fastest Way to Better Results
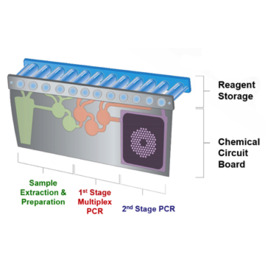
The FILMARRAY is an FDA-cleared multiplex PCR system that integrates sample preparation, amplification, detection and analysis. It requires just a few minutes of hands-on-time and its turnaround time is just about an hour, giving you faster results which may lead to better patient care.
The FILMARRAY now has four FDA-cleared panels – the Respiratory Panel, the Blood Culture Identification Panel, the Gastrointestinal Panel, and the Meningitis/Encephalitis Panel. Together, these panels test for more than a hundred pathogens. Thus, FILMARRAY is not only the fastest way to better results; it’s the fastest way to more results.
BIOFIRE FILMARRAY® FEATURES
Set It Up In 2 Minutes
With minimal training and no precise pipetting required, in as little as 2 minutes, the patient sample is loaded into the pouch and placed into the system for an automated test.
Simplicity Is In The Software
The FILMARRAY Software walks you through the entire process—from sample to answer. When the run is complete, the software analyzes and reports results in a simple, easy-to-read format.
BIOFIRE FILMARRAY® USES
One Streamlined System
Many Applications
Diagnosing infectious diseases can be challenging. There are often countless possibilities and finding out what is making a patient sick often requires rapid answers so appropriate treatment decisions can be made. The FILMARRAY is an easy-to-use multiplex PCR system that can be used in complex diagnostic situations that require multiple answers in a critically short period of time:
- Upper respiratory tract infections
- Identification of organisms in positive blood cultures
- Gastrointestinal infections
- Meningitis/Encephalitis
The technology behind the FILMARRAY allows for the simultaneous detection of:
- Viruses
- Bacteria
- Yeast
- Parasites
- Antimicrobial resistance genes
Whether trying to select appropriate therapy for a septic patient, or determining exactly which respiratory pathogen is making a young child sick, FILMARRAY can return answers fast.
BIOFIRE FILMARRAY® REAGENTS
Respiratory Panel: FDA Cleared
The FILMARRAY Respiratory Panel tests for a comprehensive panel of 20 respiratory viruses and bacteria. The FILMARRAY instrument integrates sample preparation, amplification, detection and analysis into one simple system that requires 2 minutes of hands-on time and has a total run time of about 1 hour.
- Simple: 2 minutes of hands-on time
- Easy: No precise measuring or pipetting required
- Fast: Turnaround time of about 1 hour
- Comprehensive: 20 target respiratory panel
|
Viral Targets |
||
|
Adenovirus |
Coronavirus HKU1 |
Coronavirus NL63 |
|
Coronavirus 229E |
Coronavirus OC43 |
Human Metapneumovirus |
|
Human Rhinovirus/Enterovirus |
Influenza A |
Influenza A/H1 |
|
Influenza A/H3 |
Influenza A/H1-2009 |
Influenza B |
|
Parainfluenza Virus 1 |
Parainfluenza Virus 2 |
Parainfluenza Virus 3 |
|
Parainfluenza Virus 4 |
Respiratory Syncytial Virus |
|
|
Bacterial Targets |
|
Bordetella pertussis |
|
Chlamydophila pneumoniae |
|
Mycoplasma pneumoniae |
Gastrointestinal (GI) Panel: FDA Cleared
The FILMARRAY Gastrointestinal (GI) Panel tests for common gastrointestinal pathogens including viruses, bacteria and parasites that cause infectious diarrhea. The integrated FILMARRAY system brings sample to results in about an hour, with only 2 minutes of hands-on time.
- Simple: 2 minutes of hands-on time
- Easy: No precise measuring or pipetting required
- Fast: Turnaround time of about 1 hour
- Comprehensive: 22 target GI panel
|
Viruses |
|
|
Adenovirus F40/41 |
Astrovirus |
|
Norovirus GI/GII |
Rotavirus A |
|
Sapovirus (I, II, IV, and V) |
|
|
Bacteria |
|
|
Campylobacter (jejuni, coli and upsaliensis) |
Clostridium difficile (Toxin A/B) |
|
Plesiomonas shigelloides |
Salmonella |
|
Yersinia enterocolitica |
Vibrio (parahaemolyticus, vulnificus and cholerae) |
|
Vibrio cholerae |
Diarrheagenic E.coli/Shigella |
|
Enteroaggregative E. coli (EAEC) |
Enteropathogenic E. coli (EPEC) |
|
Enterotoxigenic E. coli (ETEC) lt/st |
Shiga-like toxin-producing E. coli (STEC) stx1/stx2 |
|
E. coli O157 |
Shigella/Enteroinvasive E. coli (EIEC) |
|
Parasites |
|
|
Cryptosporidium |
Cyclospora cayetanensis |
|
Entamoeba histolytica |
Giardia lamblia |
Blood Culture Identification (BCID) Panel: FDA Cleared
The FILMARRAY Blood Culture Identification (BCID) Panel tests for a comprehensive list of 24 pathogens and 3 antibiotic resistance genes associated with bloodstream infections. With just one test you can identify pathogens in 9 out of 10 positive blood cultures in about an hour with only 2 minutes of hands-on time.
- Simple: 2 minutes of hands-on time
- Easy: No precise measuring or pipetting required
- Fast: Turnaround time of about 1 hour
- Comprehensive: 27 target BCID panel
|
Bacteria |
|
|
Gram-positive bacteria |
|
|
Enterococcus |
Listeria monocytogenes |
|
Staphylococcus |
Streptococcus |
|
Staphylococcus aureus |
Streptococcus agalactiae |
|
Streptococcus pneumoniae |
Streptococcus pyogenes |
|
Gram-negative bacteria |
|
|
Acinetobacter baumannii |
Haemophilus influenzae |
|
Neisseria meningitidis |
Pseudomonas aeruginosa |
|
Enterobacteriaceae |
Enterobacter cloacae complex |
|
Escherichia coli |
Klebsiella oxytoca |
|
Klebsiella pneumoniae |
Proteus |
|
Serratia marcescens |
|
|
Yeast |
|
Candida albicans |
|
Candida glabrata |
|
Candida krusei |
|
Candida parapsilosis |
|
Candida tropicalis |
|
Antimicrobial resistance genes |
|
mecA - methicillin resistance |
|
vanA/B - vancomycin resistance |
|
KPC - carbapenem resistance |
Meningitis/Encephalitis (ME) Panel: FDA Cleared
The FILMARRAY Meningitis/Encephalitis (ME) Panel tests cerebrospinal fluid (CSF) for a variety of pathogens including bacteria, viruses, and yeast. The integrated FILMARRAY system yields sample to results in about an hour, with only 2 minutes of hands-on time.
- Simple: 2 minutes of hands-on time
- Easy: No precise measuring or pipetting required
- Fast: Turnaround time of about 1 hour
- Comprehensive: 16 bacterial, viral, and yeast targets
|
Bacteria |
|
|
Escherichia coli K1 |
Haemophilus influenzae |
|
Listeria monocytogenes |
Neisseria meningitidis |
|
Streptococcus agalactiae |
Streptococcus pneumoniae |
|
Viruses |
|
|
Cytomegalovirus (CMV) |
Enterovirus |
|
Epstein-Barr virus (EBV) |
Herpes simplex virus 1 (HSV-1) |
|
Herpes simplex virus 2 (HSV-2) |
Human herpesvirus 6 (HHV-6) |
|
Human parechovirus |
Varicella zoster virus (VZV) |
|
Yeast |
|
Cryptococcus gattii |
|
Cryptococcus neoformans |