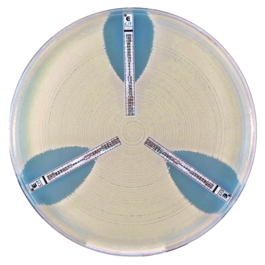
ETEST® Ceftolozane/ Tazobactam
Improving Therapeutic Decisions
ETEST is a well-established method for antimicrobial susceptibility testing in microbiology laboratories around the world. ETEST consists of a predefined gradient of antibiotic concentrations on a plastic strip and is used to determine the Minimum Inhibitory Concentration (MIC) of antibiotics, antifungal agents and antimycobacterial agents. Learn more about ETEST.
Now FDA 510(k) Cleared!
ETEST Ceftolozane/Tazobactam
(CT 0.016-256 µg/mL)
For More
Information
Optimizing antimicrobial therapy in the face of growing antimicrobial resistance
Multi-drug Resistant Organisms (MDRO) are a serious and growing healthcare threat. Ensuring that patients get the right antimicrobial, in the right dose, at the right time is at the heart of antimicrobial stewardship. This helps improve patient outcomes, prevents the development of resistance, and preserves the effectiveness of new therapeutic molecules. Vigilant and accurate antimicrobial susceptibility testing is a critical part of fighting resistance.
ETEST Ceftolozane/Tazobactam (C/T 0.016-256 µg/mL)
ETEST C/T (0.016-256 µg/mL) strips are easy-to-use and provide a wealth of information to clinicians. ETEST generates MIC values from a continuous scale and can give results between conventional two-fold dilutions, i.e., half dilutions. This means that, unlike diffusion methods, they can provide exact MIC breakpoints as well as the interpretive category (S, I, R). Not only do you know if an organism is susceptible, but you know with great precision how susceptible – information that can help best guide antibiotic therapy to fight the infection while reducing the risk of development of drug-resistant bacteria. Precise MIC values also enable epidemiological tracking of evolution of infectious organisms, to detect emergence of resistance over time.
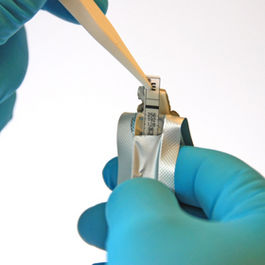
The thin, inert and non-porous plastic ETEST C/T (0.016-256 µg/mL) strips have the MIC reading scale in μg/mL on one side and a predefined antibiotic gradient on the other. After applying a strip to an inoculated agar surface, the antibiotic gradient transfers into the agar and will form a stable, continuous and exponential gradient of antibiotic concentration is formed directly under the strip. After incubation, whereby bacterial growth becomes visible, a symmetrical inhibition ellipse centered along the strip is seen. With as little as 16 hours incubation, you can read the MIC value on the scale, with complete inhibition seen where the pointed end of the ellipse intersects the strip. It’s that simple.
Zerbaxa®,† a combination of ceftolozane and tazobactam, is used for Enterobacteriaceae and Pseudomonas aeruginosa infections. It is indicated for the treatment of adult patients with complicated urinary tract infections (cUTI) including acute pyelonephritis, and in combination with metronidazole, complicated intraabdominal infections (cIAI).
ETEST C/T (0.016-256 µg/mL) strips offer an easy and reliable method to determine exact MIC values. Susceptibility testing to Ceftolozane and Tazobactam help ensure optimal treatment and follow-up.
Content of the Kit
Single Pack: 30 test strips
-
Ceftolozane MIC range: 0.016 - 256 μg/mL
-
Tazobactam: 4 μg/mL
Ordering Contact Information
Customer Service: (800) 682-2666
To order please visit:
our eCommerce store
ETEST Application Guide
For information about the many uses of ETEST, by organism, download the ETEST Application Guide here.
ETEST Technical Library
- Please navigate to Technical Library at top left or at www.mybiomerieux.com (requires login).
- To access ETEST related documents, select Guided Search by a reagent;
- Click the “+” by ETEST; the desired ETEST product area, for example “Antimicrobial Susceptibility Testing”.
- In the ensuing list of Available Products, select the product of interest.
- The ETEST package insert is Package Insert US ‐ 15203 ‐ x ‐ en ‐ ETEST ‐ AST.pdf. Supplementary Inserts specific to each antimicrobial are also available.
- For the ETEST Application Guide, select Guided Search by reagents; click the Search by product box, enter “410440 -ETEST Additional Technical Documents and Search Instructions”, then “Supplementary Inserts ‐ 16273 ‐ A ‐ en ‐ EAG ‐ ETEST Application Guide.pdf”.
For new users, please request a login; logins are returned during business hours.
References:
1. ETEST C/T (0.016-256 µg/mL) package insert, available at http://www.mybiomerieux.com. Instructions on accessing the Technical Library may be found here.
2. Zerbaxa® official site: http://www.zerbaxa.com/
†Zerbaxa is a trademark belonging to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Downloads
ETEST Publications
Pseudomonas aeruginosa and Enterobacteriaceae:
ASM Microbe 2017 - Performance of ceftolozane-tazobactam ETEST for antimicrobial susceptibility testing of Pseudomonas aeruginosa and Enterobacteriaceae. University of California, Los Angeles, CA. Poster #: Friday - 424.