What is Antimicrobial Resistance?
If we do not address antimicrobial resistance (AMR) now, we will lose the ability to safely perform routine procedures and surgeries. Antimicrobial resistance occurs when bacteria, fungi, viruses, and parasites mutate naturally over time and no longer respond to modern medicines. This means that standard antibiotic treatments become ineffective, and infections persist, increasing the risk of severe consequences to the patient.
High Cost of Antimicrobial Resistance
- 1.27 million deaths were directly attributed to antimicrobial resistance according to the 2019 data published in The Lancet1
- In 2017, the CDC reported an estimated economic burden of $4.6 billion annually in the U.S., associated with treating six alarming antibiotic resistance threats.2
The Best Solution to Fight Antimicrobial Resistance
Antimicrobial stewardship (AMS) is a commitment to preserving antibiotic efficacy for future generations through appropriate use of antimicrobial drugs.The goal of antimicrobial stewardship initiatives is to ensure that the right antibiotic is given to the right patient at the right time, with the right dose and via the right route—causing the least harm to the patient. In realistic terms, it is a multidisciplinary approach that aims to ensure that patients benefit from the most effective antibiotic treatments, while limiting the side effects and costs of unnecessary treatments.
bioMérieux - Your Partner of Choice for Antimicrobial Stewardship
bioMérieux is your trusted partner in today's healthcare systems. We provide the most comprehensive diagnostics & expertise, enabling hospital laboratories to provide actionable evidence & insights to clinicians, partnering together on improving patient outcomes and creating more effective stewardship programs.
Integrated Offer
Our end-to-end antimicrobial stewardship solution encompasses more than just traditional state-of-the-art lab technology. Because at bioMérieux we fully understand your antimicrobial stewardship (AMS) needs, our offer translates into 3 key areas where we help hospitals and labs worldwide evolve to suit today’s needs and tomorrow’s concerns.
|
ACTIONABLE DIAGNOSTICS: Diagnostic tools help clinicians determine timely and appropriate treatment which help to reduce the spread of antimicrobial resistant infections. As the largest in vitro diagnostics company committed to antimicrobial stewardship, we help maximize the use of diagnostic solutions to detect, identify, and treat today's most urgent drug-resistant pathogens. |
|
|
ADVANCED ANALYTICS: We support healthcare systems in their efforts to deliver evidence-based clinical care with our software solutions that help to optimize efficiency and increase collaborative communication. |
|
|
COLLABORATIVE SERVICES: Improve lab efficiency and accelerate the medical and economic value of stewardship. |
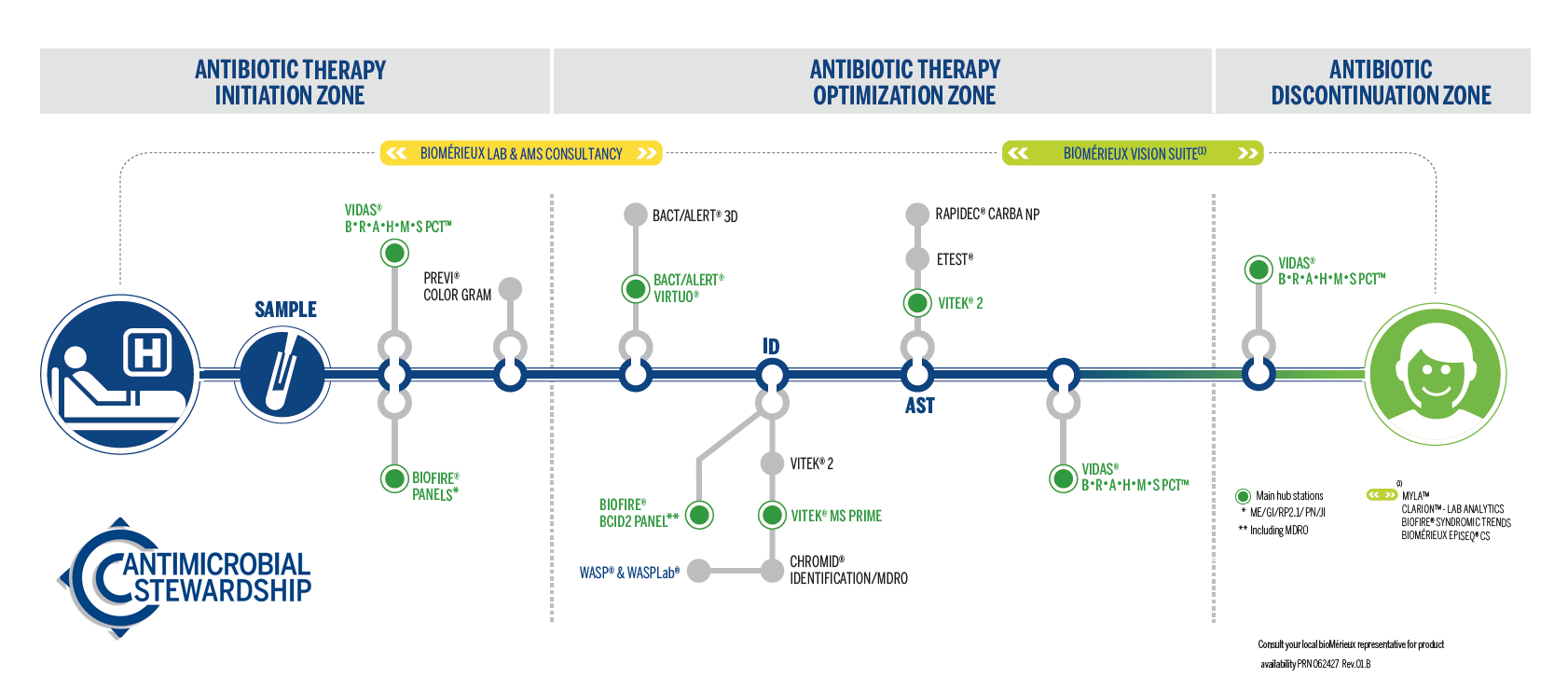
Complete Antimicrobial Stewardship Solution by Patient Pathway
bioMérieux has the most comprehensive solutions on the market all along the patient pathway.
Diagnostics are at the heart of antimicrobial stewardship helping drive informed therapy decisions from antibiotic initiation to optimization and discontinuation.
Antibiotic Therapy Initiation
Our solutions confirm or rule out bacterial infection and identify the causative pathogen for informed patient management.
Antibiotic Therapy Optimization
Optimize antibiotic therapy guidance and improve patient outcomes faster with our diagnostic solutions that help identify pathogens and determine their resistance profiles in order to select the most appropriate treatment.
Antibiotic Discontinuation
VIDAS B•R•A•H•M•S PCT aids in decision-making for antibiotic therapy discontinuation for patients with suspected or confirmed sepsis, and for patients with lower respiratory tract infection (LRTI).
Our expertise in microbiology and infectious diseases, coupled with our collaborative Lab Optimization Consultancy and Stewardship Consultancy practices, enables us to help our partners understand their real processes—then visualize, analyze, and apply data, to support positive patient outcomes. Our partnership approach reveals best practices that can improve patient care, and contribute to economically sustainable healthcare systems by creating ways to maintain lower costs.
Lab Optimization Consultancy
With our patient-centric approach, we help laboratories optimize and transform their workflow to support clinical decisions and better patient outcomes. Relevant and timely results have become key in the race to be more efficient and control costs. This elevates the important role the laboratory plays in the value-based healthcare model.
DOWNLOAD THE LAB OPTIMIZATION BROCHURE
Stewardship Consultancy
Our customer-centric approach, teamed with our extensive knowledge and experience, means that we see things from a unique angle and can provide consultancy to support stewardship programs with cross-departmental impact. We help hospitals execute and improve antimicrobial stewardship through actionable road maps and discovery of dynamic insights.
Antimicrobial Stewardship Centers of Excellence
bioMérieux is taking a proactive, partnership-driven approach by creating the Antimicrobial Stewardship Centers of Excellence (COE) initiative to focus on delivering high-value solutions, expertise and service in antimicrobial stewardship (AMS) efforts in order to improve patient care.
AMS Centers of Excellence Partnerships
In 2021, bioMérieux established the Antimicrobial Stewardship Centers of Excellence program to bring attention to the threat of antimicrobial resistance and to accelerate the impact that infectious diseases diagnostics have in facilitating antimicrobial stewardship and better patient care. bioMérieux has formalized partnerships with 10 hospitals globally, including sites in the United States, India, China, Malaysia, Morocco, France, Italy, Colombia, and Chile. These sites are leaders in their countries and regions in regards to integrating diagnostics into antimicrobial stewardship. The partnerships will focus on generating and showcasing real-world medical and economic data and best practices about the value of combining diagnostics, medical education, lab consultancy services, and information technology solutions.
AMS Centers of Excellence News and Highlights
bioMérieux and the AMS Centers of Excellence have a shared vision for the future of managing antimicrobial resistance and improving antimicrobial stewardship initiatives. This designation signifies these hospitals and laboratories as partners of choice for bioMérieux to further define and demonstrate data-driven best practices, to improve interventions and identify new diagnostic solutions in combatting antimicrobial resistance.
In North America, Tampa General Hospital and Henry Ford Health are two health systems that have received this Global Antimicrobial Stewardship Center of Excellence designation. Find out more as their team shares what this designation means to them.
Evidence
|
Data Analytic Platform Provides Insights for Reflexive Urine Culture Implementation at an Academic Medical Center CLARION, a bioMérieux data analytic platform, impacts the framework for a standardized reflexive urine culture program that could reduce diagnostic workload and potentially minimize antibiotic overutilization. |
Stewardship Resources
External Partner Resources
 As a proud member of the Antimicrobial Resistance Industry Alliance, we invite you to view the 2021 Progress Report which provides a snap shot of the life sciences industry's collective efforts and leadership in tackling the rise of antimicrobial resistance.
As a proud member of the Antimicrobial Resistance Industry Alliance, we invite you to view the 2021 Progress Report which provides a snap shot of the life sciences industry's collective efforts and leadership in tackling the rise of antimicrobial resistance.
 bioMérieux is proud to be a co-leader in the VALUE-Dx project, a consortium of 26 partners which aims to demonstrate the medical and economic value of diagnostics to combat antibiotics resistance.
bioMérieux is proud to be a co-leader in the VALUE-Dx project, a consortium of 26 partners which aims to demonstrate the medical and economic value of diagnostics to combat antibiotics resistance.
 bioMérieux was selected as a supplier in a tender process by the Fleming Fund, a £265 million UK aid investment to tackle antimicrobial resistance in low- and middle-income countries around the world.
bioMérieux was selected as a supplier in a tender process by the Fleming Fund, a £265 million UK aid investment to tackle antimicrobial resistance in low- and middle-income countries around the world.
 In November 2019, the Centers for Disease Control updated the Core Elements of Hospital Antibiotic Stewardship Programs to reflect key learnings and evidence, further advancing the viability of stewardship programs in the United States.
In November 2019, the Centers for Disease Control updated the Core Elements of Hospital Antibiotic Stewardship Programs to reflect key learnings and evidence, further advancing the viability of stewardship programs in the United States.
![]() bioMérieux supports CIDRAP with grants for the Antimicrobial Stewardship Project (ASP). We encourage you to visit the Center for Infectious Disease Research and Policy's ASP page to view the latest industry news and updates about stewardship in the United States.
bioMérieux supports CIDRAP with grants for the Antimicrobial Stewardship Project (ASP). We encourage you to visit the Center for Infectious Disease Research and Policy's ASP page to view the latest industry news and updates about stewardship in the United States.
CONTACT US ABOUT OUR AMS SOLUTIONS
References:
- Murray CJ, Ikuta KS, Sharara F, et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. The Lancet. 2022;399(10325):629-655. doi:https://doi.org/10.1016/S0140-6736(21)02724-0
- Nelson RE, Hatfield KM, Wolford H, et al. National Estimates of Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clinical Infectious Diseases. 2021;72(Supplement_1):S17-S26. doi:https://doi.org/10.1093/cid/ciaa1581
PRN 066185 REV 02.A