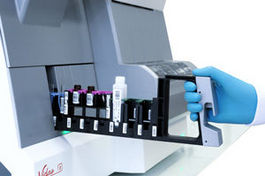
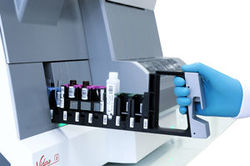
VIDAS® 3
Compact immunoanalyzer with full traceability and automation
VIDAS 3 is a fully automated benchtop immunoassay system based on the proven enzyme-linked fluorescent assay (ELFA) technology, for high-quality on-demand test results.
- Specialty menu available in single-test ready-to-use format
- Bidirectional connectivity to internal and external networks
- Robust system: MTBF > 370 days
VIDAS 3 is the new-generation VIDAS analyzer, a brand trusted around the world in immunoassay testing for over three decades. Designed with your lab’s specific needs and workflow in mind, it is equipped with increased automation, traceability and connectivity for greater productivity. VIDAS 3 helps you provide precise and accountable test results for the benefit of clinicians and their patients.
Test Security
- Reduced hands-on time from the sample to the result
- Fully automated pipetor action with single-use, disposable tips to avoid contamination and sample-to-sample carry-over
- Automated calibration and on-board dilution
- Scanning of sample and reagent barcodes upon test loading for complete traceability of all test components
- Integrated quality control management
Lab Productivity
- Facilitated lab workflow with a simple answer to complex needs
- Calibration every 14, 28 or 56 days, depending on the parameter
- Bi-directional LIS connectivity
- Minimal maintenance, allowing operators to concentrate on higher-level tasks
- Remote access via screen-sharing
Continual Access
- Immediate start, no waiting
- Temperature-controlled loading bay
- 3 separate racks each containing 3 configurable segments can hold a total of 27 samples, diluents and calibrators
- 4 independent compartments can each run 3 tests
- Up to 36 tests per hour
- Specific management of emergency tests
Fast Adoption

- Intuitive touchscreen interface
- Streamlined test loading, ensuring standardization within your lab
- Minimal training necessary before starting routine operations
Specialized Test Menu
- Specialty menu in single-test format for the diagnosis of sepsis, infectious diseases, and pregnancy
- On-demand testing: 1 patient, 1 test, 1 result
- Ready-to-use reagents
- All components needed for the test included in the kit: only 1 order reference per test
- Rapid results: from 17 to 90 minutes
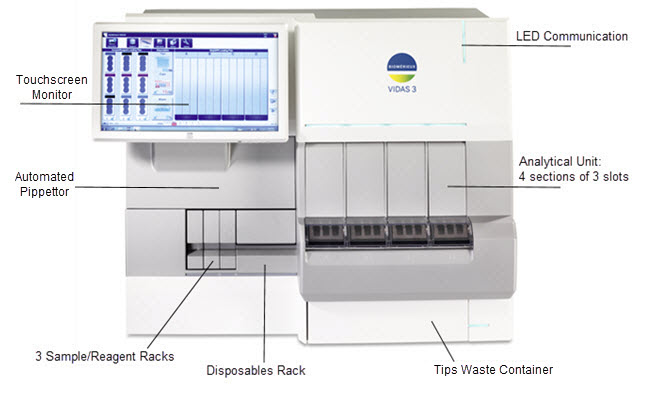
Instrument Overview
Technical Specifications
| Weight | 143 lbs (65 kg) |
| Voltage | 100-240 VAC |
| Electrical consumption | 1.2 - 3 A |
| Frequency | 50-60 Hz |
| Power | 280 VA maximum |
| Heat emission | approximately 921 BTU/hr |
| Maximum altitude | 2,500 m |
| Humidity | Relative humidity : 20 to 80% (RH non-condensing) |
| Noise | Standby mode: 55dB (A) During Analysis : 64.5 dB(A) |
Instrument Dimensions
Pre-Analytical Unit
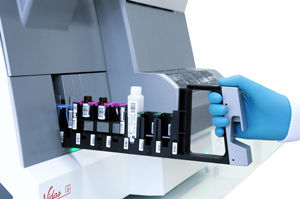

- Fully automated pippetor with single-use, disposable tips to avoid contamination
- 3 configurable racks to load up to 27 patient samples, controls, calibrators and diluents
- Disposables rack for tips and dilution cups
Analytical Unit

- 4 sections to allow continual access
Software
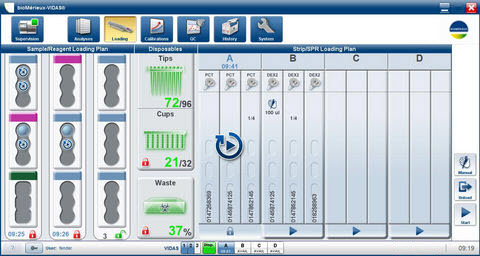
- Easy-to-use software program for guided instrument operation, reporting and troubleshooting
Please contact your local bioMérieux representative for more information.
VIDAS Systems
VIDAS systems use the ELFA assay principle. This proprietary technology ensures excellent sensitivity and specificity by combining the ELISA test method with a final blue fluorescent reading.
SPR Device
 The SPR (Solid Phase Receptacle) is coated with antigens or antibodies and acts as a pipetting device.
The SPR (Solid Phase Receptacle) is coated with antigens or antibodies and acts as a pipetting device.
At each stage of the reaction, the SPR aspirates the reagents in and out. This original concept prevents any inter-reagent or inter-sample contamination and the absence of tubing, syringes, or needles minimizes system maintenance.
Reagent Strip
 The strip contains all of the reagents required for the reaction. It consists of 10 wells covered with a labeled, foil seal. The foil of the first well is perforated to facilitate the introduction of the sample. The last well of each strip is a cuvette where the fluorometric reading is performed. The wells in the center section of the strip contain the various reagents required for the assay.
The strip contains all of the reagents required for the reaction. It consists of 10 wells covered with a labeled, foil seal. The foil of the first well is perforated to facilitate the introduction of the sample. The last well of each strip is a cuvette where the fluorometric reading is performed. The wells in the center section of the strip contain the various reagents required for the assay.
Select Assays
 |
 |
||||
| VIDAS® B•R•A•H•M•S PCT™ | VIDAS® D-DIMER EXCLUSION™ II | VIDAS® LYME IgG II & VIDAS® LYME IgM II | VIDAS® C. difficile Panel |
VIDAS 3 Complete Menu of Assays
>> Download the VIDAS 3 Assay Reference Sheet
|
ASSAY NAME |
PRODUCT NUMBER |
REPORTABLE RANGE |
COMPATIBILITY* |
CPT CODE (MODIFIER) |
|
Critical and Emergency Care |
||||
|
NEW! VIDAS NEPHROCHECK |
421172-01 | AKIRISKTM SCORE | N/A | N/A |
|
VIDAS B•R•A•H•M•S PCT (Procalcitonin) |
30450-01 |
0.05-200 ng/ml |
N/A |
84145 |
|
VIDAS D-DIMER EXCLUSION II |
30455-01 |
45-10,000 ng/ml |
N/A |
85379 |
|
Infectious Diseases |
||||
|
VIDAS Measles IgG |
30219 |
Qualitative |
1 |
86765 |
|
VIDAS Mumps IgG |
30218 |
Qualitative |
1 |
86735 |
|
VIDAS Rubella IgG |
30226 |
0-250 IU/ml |
2 |
86762 |
|
VIDAS Varicella IgG |
30217 |
Qualitative |
1 |
86787 |
|
VIDAS Lyme IgG |
417401 |
Qualitative |
3 |
86618 |
|
VIDAS Lyme IgM |
416436 |
Qualitative |
3 |
86618 |
|
VIDAS C. difficile Toxin A&B (CDAB) |
30118-01 |
Qualitative |
N/A |
87324 |
|
VIDAS C. difficile GDH |
30125-01 |
Qualitative |
N/A |
87449 |
|
VIDAS H. pylori |
30192-01 |
Qualitative |
2 |
86677 |
|
VIDAS Toxo IgG |
30210-01 |
0-300 IU/ml |
2 |
86777 |
|
VIDAS Toxo IgM |
30202-01 |
Qualitative |
2 |
86778 |
|
VIDAS CMV IgG |
30204-01 |
0-400 AU/ml |
2 |
86644 |
|
VIDAS CMV IgM |
30205-01 |
Qualitative |
N/A |
86645 |
|
Reproductive Hormones |
||||
|
VIDAS HCG |
30405-01 |
2-1500 mlU/ml |
N/A |
84702 |
*Matching numbers indicate VIDAS assays that can be run using the same protocol on the VIDAS instrument.
VIDAS 3 Instrument Services
- An extensive network of skilled Systems Engineers and Application Specialists for on-site instrument maintenance
- Remote support services via screen-sharing for immediate solutions
Certification Support and Integrated Quality Control
- VIDAS validation package for your instrument and methodology
- Support in obtaining ISO 15189 certification
Training
- On-site and off-site training sessions
- E-learning courses
- A wide offer to continually develop your lab’s skills and expertise
- Access to comprehensive online learning modules through www.biomerieuxuniversity.com
Technical Documents and Resources
- To have access to more resources and strengthen your expertise, visit the resource center at resourcecenter.biomerieux.com.
PRN 059330 Rev 04.A